中国组织工程研究 ›› 2018, Vol. 22 ›› Issue (2): 210-215.doi: 10.3969/j.issn.2095-4344.0008
• 药物控释材料 drug delivery materials • 上一篇 下一篇
不同粒径重组人骨形态发生蛋白2/聚乳酸-聚羟基乙酸共聚物微球制备及体内外释放性能比较
鲍玉成1,王 勇1,张文龙1,谢 祎1,于美丽2
- 1天津市海河医院,天津市 300350;2天津市第三中心医院,天津市人工细胞重点实验室,天津市 300170
Recombinant human bone morphogenetic protein 2/poly(lactic-co-glycolic acid) copolymer microspheres with different particle sizes: preparation and release performance in vivo and in vitro
Bao Yu-cheng1, Wang Yong1, Zhang Wen-long1, Xie Yi1, Yu Mei-li2
- 1Tianjin Haihe Hospital, Tianjin 300350, China; 2Third Central Hospital of Tianjin, Tianjin Key Laboratory of Artificial Cells, Tianjin 300170, China
摘要:
文章快速阅读:
.jpg)
文题释义:
重组人骨形态发生蛋白2:是存在于人、动物骨骼和牙齿等组织中的一种蛋白质,能诱导间充质细胞分化为骨细胞形成新骨,是骨组织形成过程中最关键的一个调节因子。如果单独在缺损区局部应用重组人骨形态发生蛋白2 细胞生长因子,因其半衰期非常短,易被体液转运或酶类降解,不能充分有效发挥其成骨诱导活性。
聚乳酸-聚羟基乙酸共聚物:由乳酸和乙醇酸聚合而成,在体内经过三羧酸循环最终降解为二氧化碳和水。聚乳酸-聚羟基乙酸共聚物具有无毒安全、生物相容性好、易于骨细胞黏附生长及降解性能可控等优点,是制备缓释微球的理想材料,已被美国FDA批准临床应用,近年来被大量应用于控制降解释放的载体材料。
背景:应用生物可降解材料包载骨细胞生长因子制成微球缓释的技术,为生长因子的高效利用提供了可行性。
目的:制备重组人骨形态发生蛋白2/聚乳酸-聚羟基乙酸共聚物纳米和微米微球,通过体内外释放实验比较两种微球的释放行为差异。
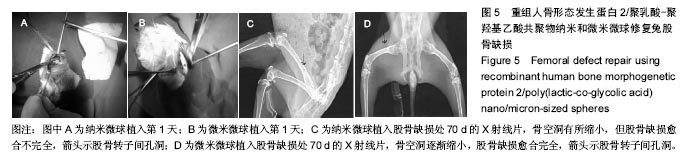
方法:通过控制匀浆速度,采用乳化-溶剂挥发法制备重组人骨形态发生蛋白2/聚乳酸-聚羟基乙酸共聚物缓释纳米和微米微球。①体外缓释实验:将两种微球分别溶于PBS中70 d,采用ELISA法检测不同时间点上清液中重组人骨形态发生蛋白2的浓度;②体内缓释实验:将44只新西兰大白兔分为2组,分别在股骨转子缺损处植入重组人骨形态发生蛋白2/聚乳酸-聚羟基乙酸共聚物缓释纳米和微米微球,植入70 d内采用ELISA法检测股骨转子缺损处重组人骨形态发生蛋白2的浓度。
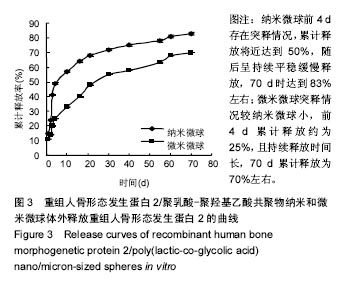
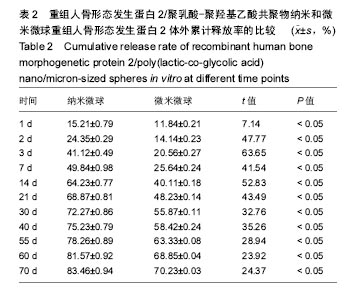
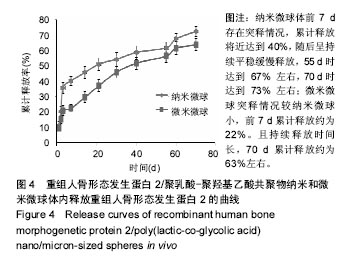
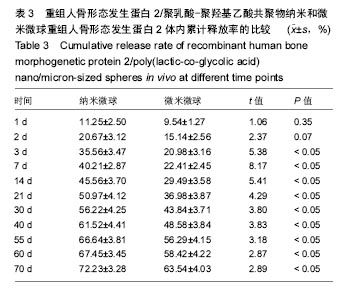
结果与结论:①体外缓释实验:纳米微球前3 d存在突释情况,累计释放将近达到41%,随后呈持续平稳缓慢释放,70 d时达到83%左右;微米微球突释情况较纳米微球小,前3 d累计释放约为20%且持续释放时间长,70 d累计释放约为70%;②体内缓释实验:纳米微球前3 d存在突释情况,累计释放将近35%,随后呈持续平稳缓慢释放,70 d时达到72%左右;微米微球突释情况较纳米微球小,前3 d累计释放约为21%且持续释放时间长,70 d累计释放约为63%左右;③结果表明:重组人骨形态发生蛋白2/聚乳酸-聚羟基乙酸共聚物微米微球缓释释放时间与骨生长周期相适宜,更有利于临床治疗骨缺损修复。
中图分类号:
.jpg)